How to get audit trail of changes to comply with 21 CFR Part 11
Case: Companies that deliver health products (pharma, medical devices) in the US must comply with special regulations. Part 11 specifies a set of guidelines to guarantee that any supplied electronic material is valid and accurate. If the file is incorrect or fake – the FDA inspection will not review it.
That means, if some change is made, you should record it.
How to ensure that your records are authentic and every change is fixed?
Get an audit log of changes with Issue history for Jira. For example, a summary of the task was changed:
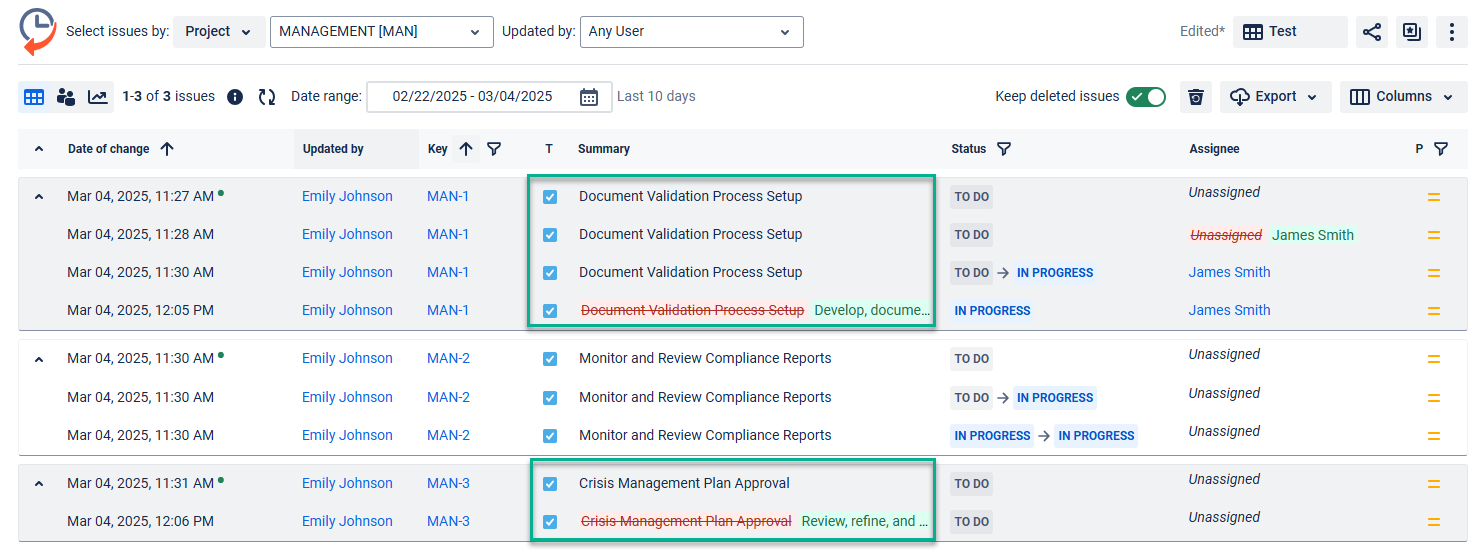
Follow the next steps to get the issues changelog:
Filter issues by Project, Assignee, Sprint, Label, etc. (depending on where you need to track changes)
Select the appropriate date range (it can be any period you need to audit your teamwork).
Add custom and standard fields to the grid using the ‘Columns’ menu. For example, the summary field.
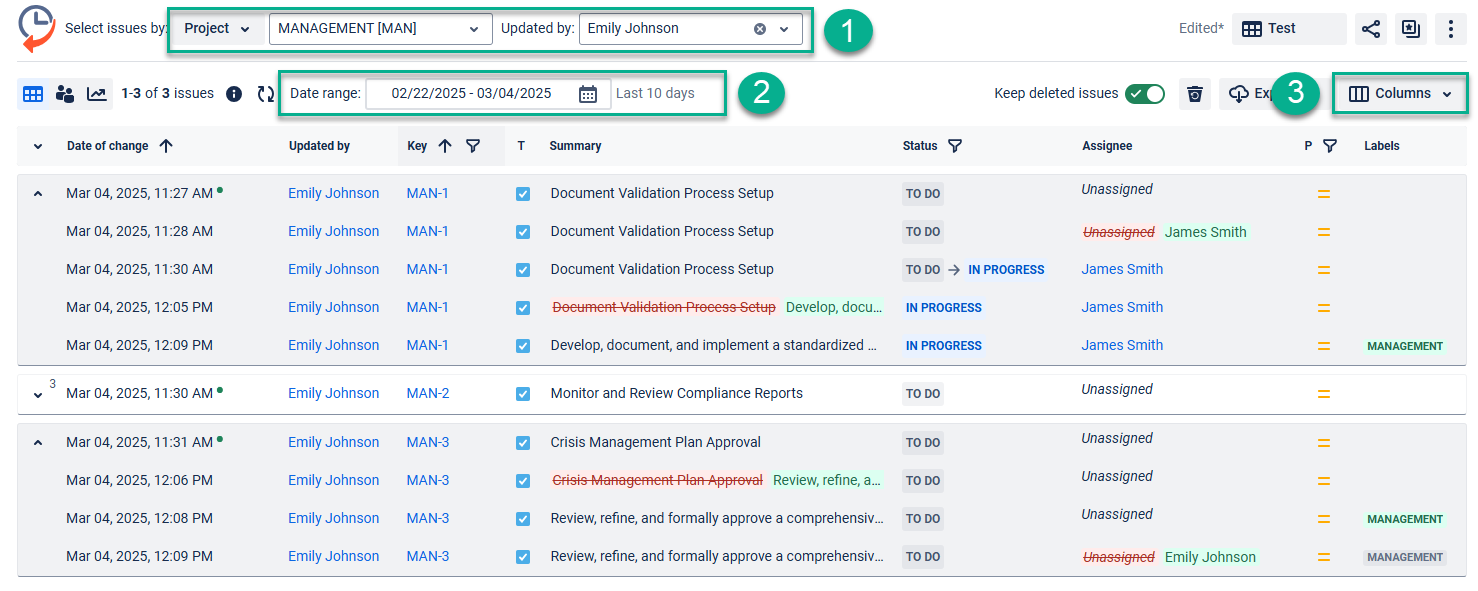
Read more here: Report generation with Issue History for Jira [Table View]
What else can you get with Issue History?
Track and keep deleted Jira issues to be sure no record is lost forever:
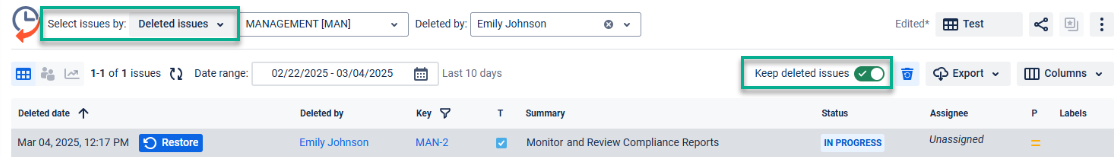
Export data from the system into a clear document (XLSX or CSV) to present it to FDA inspection:
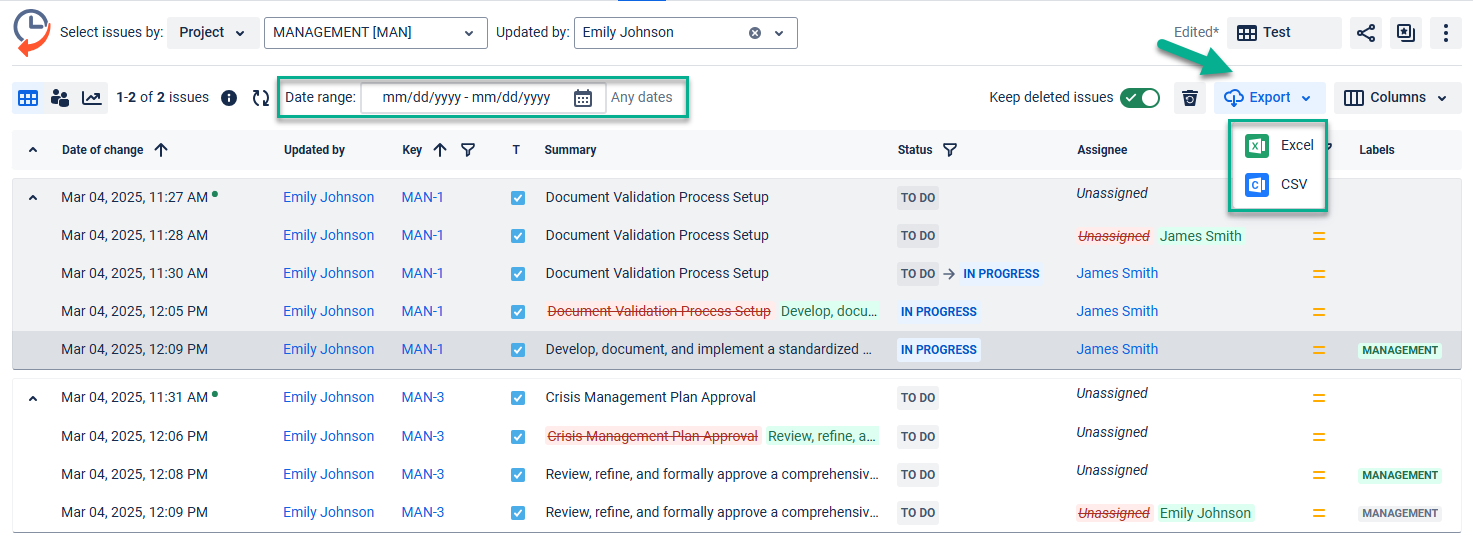
Try to get the audit log with Issue History and share your experience with us!
- Email us at support@saasjet.atlassian.net
Haven’t worked with the add-on yet? Give it a try ⬇